Yli 100 vuoden sitoutuminen tekniseen kehitykseen on tehnyt meistä johtavan valmistajan muuttuvien AC-taajuusmuuttajien, servokäyttöjen, ohjaustekniikan ja robotiikan aloilla.
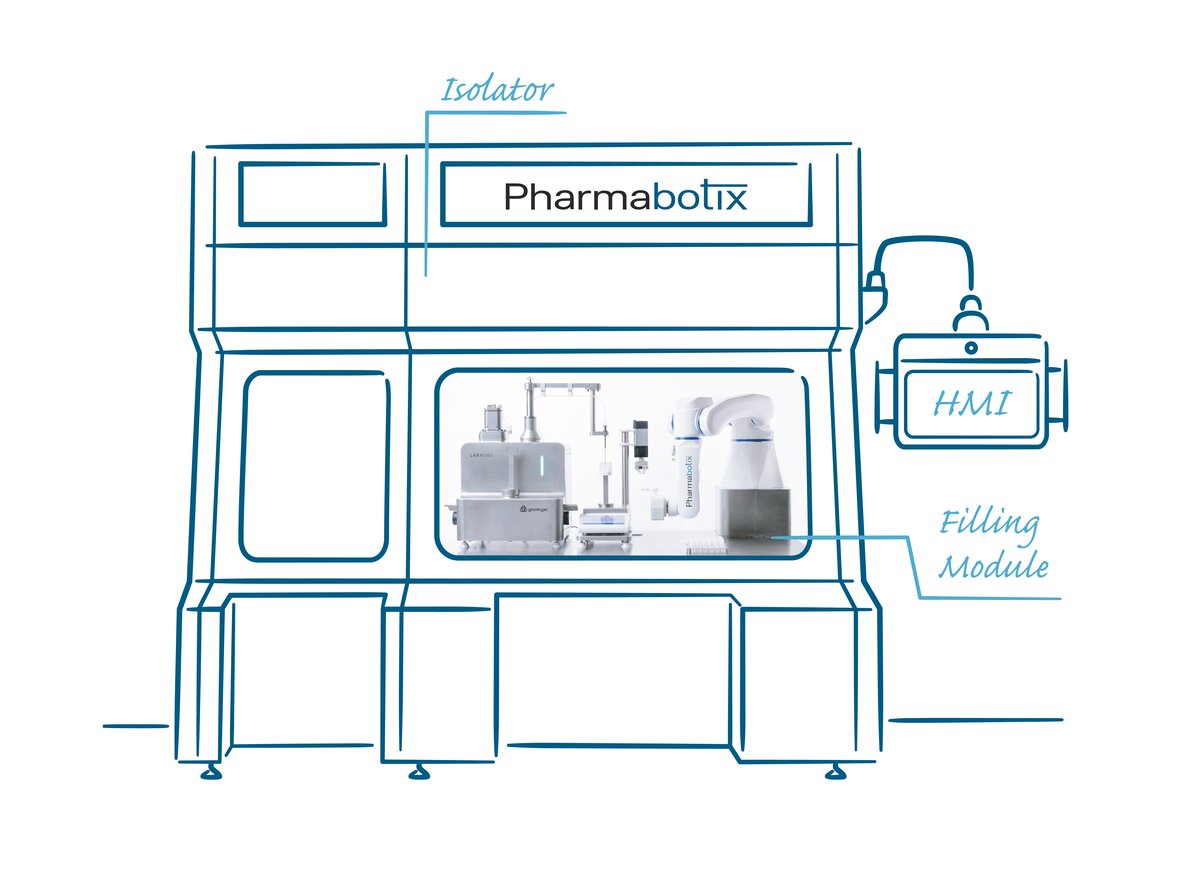
Pharmabotix uses Yaskawa’s Motoman HD8 Hygienic Design Robot for filling smaller batches
Personalized medicine – automated filling
Personalized medicine means an elementary change for the pharma and biotech industry aimed at the production of large quantities. Pharmabotix is developing automation and robotics solutions for filling smaller batches as they occur in cell and gene therapy. The Swiss company is thereby focusing on a hygienically designed Yaskawa Motoman HD8 industrial robot.
Advances in cell and gene therapy make it possible for treatments – and thus also drugs – to be individually tailored to the respective patient. These are referred to as “advanced therapy medicinal products” – in short ATMPs – or drugs for advanced therapies. Live cells or defective genes are taken from the patient, processed in the laboratory and then administered again. In this way, diseases can be treated or prevented, and even damaged tissue or organs regenerated and replaced.
“Personalized medicine means that the batch sizes of biopharmaceutical products are increasingly smaller and they are not always bottled in traditional vials or syringes,” explains Fabian Stutz, CEO of Pharmabotix. His company, which specializes in robotics and automation solutions for the pharmaceutical industry, is based in Seengen / Switzerland. Under the brand name “Sally”, Pharmabotix markets various modules for the cell and gene therapy market and lab sector.

Picture: The new machine can automatically fill up to seven vials per minute – even more in future.
Automated filling of up to seven cryovials per minute
For small batch sizes, the pharmaceutical industry relies largely on manual processes. This, however, is labour intensive. The complex process also calls for highly-trained employees, and yet the quality depends heavily on the individual. “In addition, these production processes are difficult to scale commercially and take to the mass production stage,” says Stutz.
That is why Pharmabotix has developed a concept for automated filling of cryovials for cell and gene therapy. The stipulation: the CryoFiller module must be able to automatically fill up to seven vials per minute.
Cryovials are containers made of cold-resistant plastic, in which biological samples or cells are stored in liquid nitrogen at up to -196°C. This ensures the stability and quality of the samples. Unlike classic vials, they are not closed with a plug and a metal cap, but with a screw cap.
Picture: With the CryoFiller module, Pharmabotix has developed a concept for the automatic filling of cryovials for cell and gene therapy.
The right closure on each vial
The basis of the CryoFiller is a Labworx table top system from Groninger, one of the world’s largest manufacturers of filling systems. The cryovials are placed in a rack or delivered by a flexible feeding system. One or more screw systems – depending on cycle time – open the vials. They are automatically filled and then closed again. A Yaskawa Motoman HD8 with electric gripper is responsible for handling the cryovials.
The cell itself meets the strict requirements for GMP class A and B cleanrooms and is thus classified for the manufacture of aseptic products. The cryovials are also sterile. This presented the Pharmabotix developers with a special challenge: “For the integrity of the product and seamless traceability, after filling the correct closure must be screwed onto the respective vials,” Stutz explains.
Hygienic design for the highest cleanroom class
The Pharmabotix team placed clear requirements on the robot for the CryoFiller. Among other things, cleaning should be possible with industry-standard agents and decontamination with hydrogen peroxide (H2O2). “For us only the Yaskawa’s Motoman HD8 came into consideration. We contacted Yaskawa directly, and thus we were introduced to Swiss representative SwissDrives, which provided the robot,” reports Stutz.
With the new Motoman HD series, Yaskawa recently brought two 6-axis high-performance robots onto the market that satisfy the strict demands of the pharma industry and similarly hygiene-sensitive industries. They are suitable for use in hygienic areas up to the highest cleanroom class GMP Class A.
HD stands for “hygienic design”. It was developed together with the German Fraunhofer Institute for production engineering and automation (IPA). All application cables and media lines run inside the housing. In addition, the design is rounded and free of dead spaces without external screws, gaps or undercuts. The Motoman HD8 is thus easy to clean with all standard disinfectants and procedures. The resistant surface is also particularly smooth, so that no dirt particles and microorganisms can adhere. Due to protective class IP69K it is ideally suited to lab, humid and cleanroom environments.
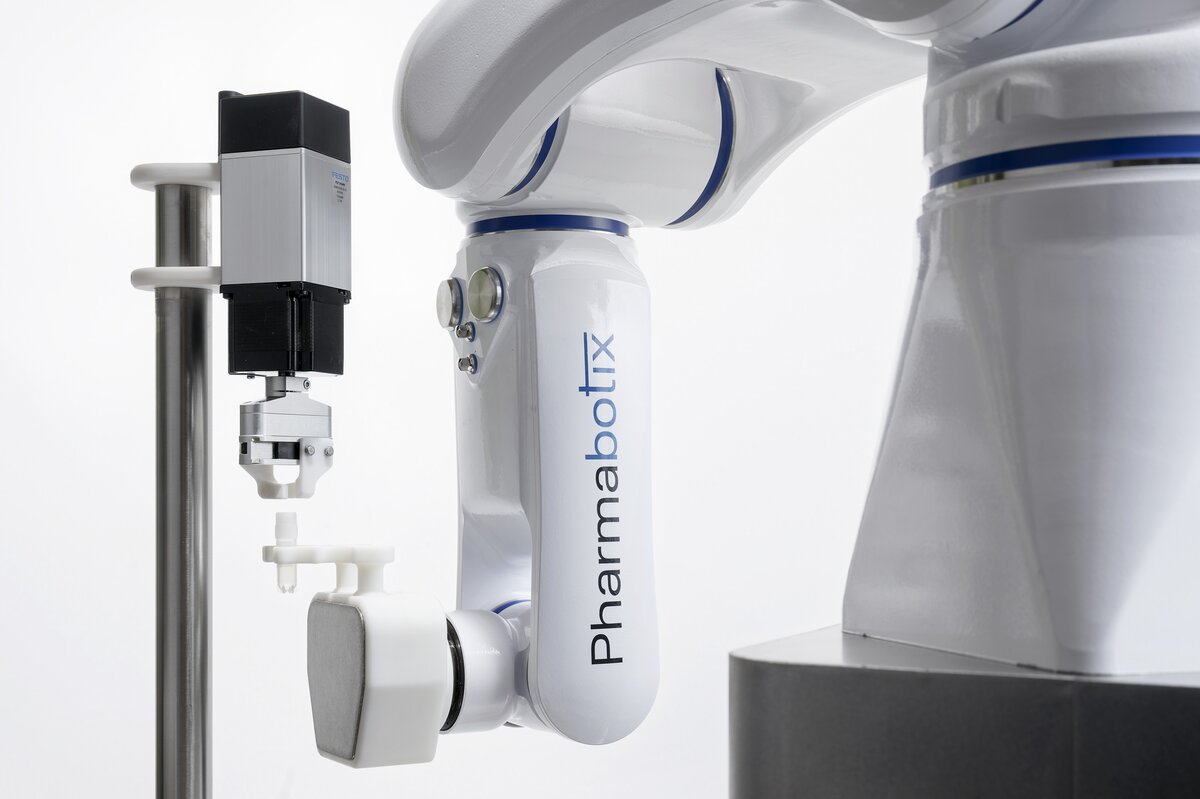
Picture: The cryovials are handled by a hygienic industrial Yaskawa Motoman HD8 robot with electric gripper.
User-friendly interface
When it comes to automation, Pharmabotix uses the SRCI Standard Robot Command Interface. This interface enables fast and straightforward programming of robot movements directly in the PLC. Without special knowledge of the robot controller, the user can navigate in the standard IEC-61131 environment and use the familiar operating environment to operate the robot. Meanwhile all genuine advantages of the robot controls are retained: the robot controls calculate the movement kinematics and guarantees high motion quality. The SRCI is not limited to a specific PLC or fieldbus.
Pharmabotix uses the Siemens WinCC unified platform. “An important prerequisite was therefore that the Motoman HD8 be compatible with the Siemens controller. Because the operation of Sally should be as simple and intuitive as possible, and achieved via a single panel. In the regulated range this offers a further advantage: Only one control must be tested – with less effort and costs,” says Stutz.

Picture: The cryovials are placed in a rack or delivered by a flexible feed system and handled by the robot.
Motoman HD8 brings us closer to the vision for Sally
The concept of the CryoFiller module for Sally was presented to potential users in a workshop as “proof of concept”. The feedback has overall been very positive. Now Pharmabotix is further developing the system which – among others – satisfies the requirements of EU GMP Annex 1. The latter defines the requirements for the manufacture of sterile pharmaceuticals in the EU.
Pharmabotix aims to position Sally as a modular platform for various different automated processes in cell and gene therapy, with robots as a central handling element. Stutz is convinced: “Together with Yaskawa and SwissDrives, and the use of a Motoman HD8 in the CryoFiller module, we have come a significant step closer to this vision.”
Source for all pictures: Pharmabotix AG
Author:
Florian Kohut, Key Account Manager Packaging Industry
Yaskawa Europe GmbH
Tel. +49-171-565-4683
florian.kohut@yaskawa.eu
www.yaskawa.de
Contact for readers’ enquiries:
Yaskawa Europe GmbH
Tel. +49-8166-90-0
robotics@yaskawa.eu
Contact Pharmabotix
Pharmabotix AG
Fabian Stutz
fabian.stutz@phamabotix.ch
www.pharmabotix.ch
Sales partner Yaskawa Switzerland:
SwissDrives AG
Tel. +41-71 844 00 88
info@swissdrives.ch



















